
Sometimes, I experience a mental block when trying to memorize certain facts. In such cases, understanding the concept first helps me work through the block. One example of such a block is whether it is possible to bond chemical-cure material to light-cure material or vice versa. There is indeed a difference between these two scenarios, and it wasn’t until I researched and understood the concept of composite polymerization that everything started to make sense and stick in my memory. So, let me explain it to you!
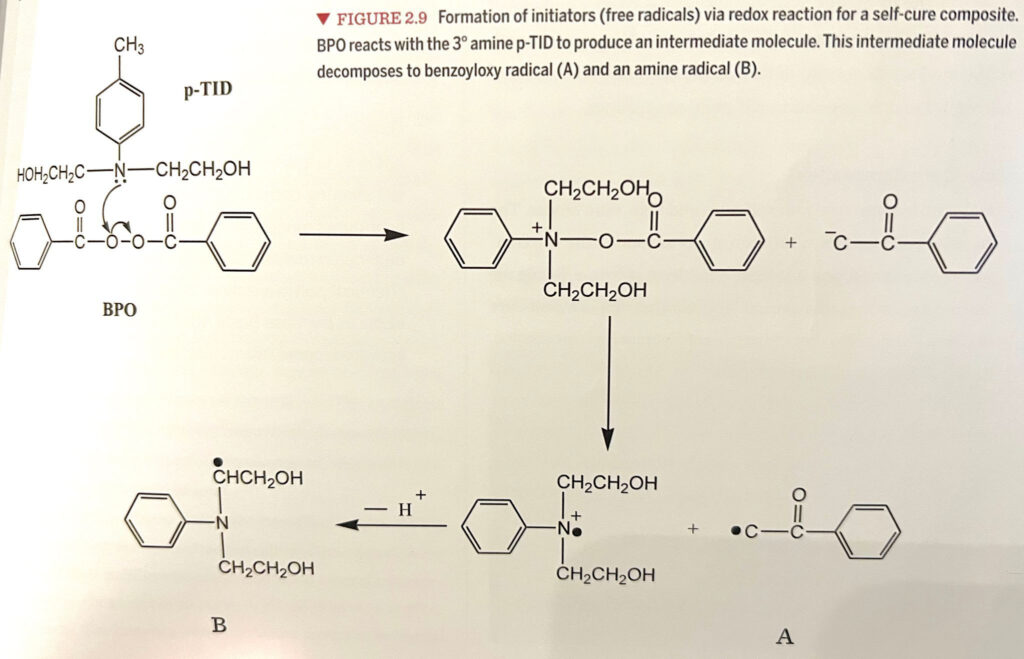
Composite polymerization occurs in four stages: Activation, Initiation, Propagation, and Termination. The most significant difference between light cure and chemical cure composites is at the activation stage. Free radicals (molecules with a free electron) must be created in a composite polymerization reaction. For a light cure composite, the wavelength of blue light activates the light initiator, usually camphoroquinone (CQ). CQ is excited and seeks out an amine co-initiator (sidekick), much like caffeine energizes us. When CQ and the amine sidekick meet, they break into a free radical molecule.
 |
A chemical cure composite is a type of composite that is polymerized without the use of light. It is typically used as build-up material in a dual syringe, which dispenses and mixes two separate materials. One syringe contains a peroxide initiator, while the other contains a catalyst amine. When these materials are mixed, the contact between these two molecules initiates a reaction that forms free radicals. The method by which these free radicals are generated allows for a light cure composite to bond with a chemical cure composite, but not vice versa.
To understand this, let’s consider how we bond composite materials together. The composite material does not convert 100% of its free monomers into chains. At most, it will convert 70%, leaving the remaining 30% free monomers available to start chains with the next composite layer. The polymer chains always have a terminal end with a double bond, which can also bond with another monomer.
When a new composite layer is added over an old one, initiators must activate to form new free radicals that will open the bonds to the free monomers. If these initiators are light-activated, more energy is involved, which helps the initiators cross into the underlying composite. However, if the composite is chemically cured, there is less energy to push these peroxides to the underlying composite and create new free radicals. This is why curing a chemical cure composite on top of a light cure layer is more difficult.
When light cannot reach the bottom of the preparation, it is best to use a chemical cure composite. For example, a light cure composite at the prep’s base is unsuitable in deep preps such as a root canal space. Instead, use a chemical cure composite at the base and a light cure on top to finish building the biobase.
But what if we are cementing a dual-cure resin cement to our prep covered with immediate dentin sealing and resin coat? Luckily, dual-cure means there are light initiators inside the cement. As long as light can reach the cement, the light initiators can start the reaction. Therefore, we don’t want a deep margin on our preparation, as it will be tough to isolate and difficult for light to penetrate. This leaves the dual-cure resin cement to polymerize through a chemical cure instead.
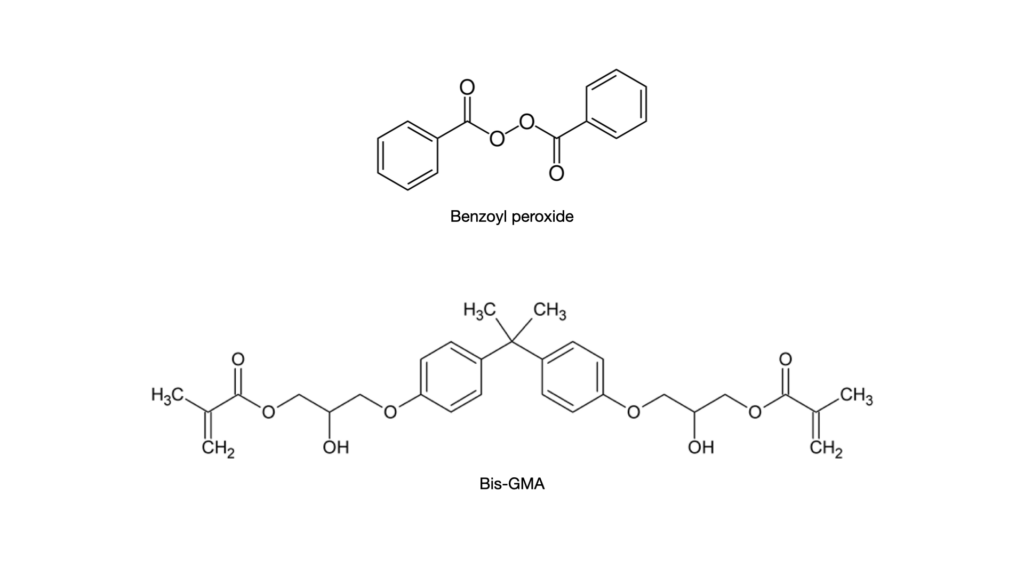
Interestingly, Bis-GMA (a common resin monomer) and the peroxide in the catalyst can also antagonistically affect each other. A chemical antagonistic effect is when two molecules react, and their product’s effect is less than either molecule individually. An example is an antidote for a poison. These two compounds undergo an antagonistic reaction to cancel out the effects of the poison. So unless properly formulated, the Bis-GMA and peroxide can cancel each other out, and the composite will not be able to polymerize in a self-cure way. So read your storage instructions for your dual cure materials. Suppose it says it needs to be refrigerated. In that case, it can indicate that this instability between Bis-GMA and benzoyl peroxide can occur in the product at room temperature, thus ruining the material’s efficacy.
When the adhesive is acidic (under a pH of 3), it has been found that the hydrogen ions in the oxygen-inhibiting layer will compete for the amine. When the hydrogen ion and amine react, the benzoyl peroxide can no longer react with the amine to create free radicals. This leads to the resin monomers being unable to form chains with the monomers in the adhesive, leading to bond failure between the self-cure composite and adhesive.
This is important information if you use a universal adhesive with a pH lower than 3 and a resin cement for indirect restorations. Certain companies will have a dual-cure activator that can be added to the adhesive to make it compatible with self-cure composites. An example is Kuraray, which has a separate dual-cure activator bottle. Other companies will put the activator in their cement to correspond to their adhesive. An example is Scotchbond Universal (the older version) with their RelyX Ultimate resin cement. Some companies do not have the tertiary amine in their resin cement, such as Kerr, with their NX3 line. This is why there is no separate dual-cure activator being sold by Kerr for their Optibond adhesives.
These adhesives must be mixed with a separate dual cure activator to become self-cure compatible. This incompatibility created the need for dual cure activators that overcome this incompatibility.
These dual cure activators contain aryl sulfinic sodium salt, which would react with the hydrogen ions from the adhesive. By removing the hydrogen ions, the pH of the adhesive increases, and the amine from the base paste will no longer react with the hydrogen, allowing it to react with the peroxide catalyst to form free radicals to start resin polymerization.

I read a paper on this subject examining adhesives, their activators, and their corresponding resin cement. This paper measured for an exothermic reaction to determine what combinations of materials would start a polymerization reaction. Interestingly, adding the activator to the adhesive did not make these adhesives self-cure.
The results were interesting because if you look at the product description of Clearfil’s DC activator, it says it will make the adhesive into a dual-cure adhesive.
Reading the manufacturer’s instructions and comprehending the science behind a product is crucial. Certain dual cure activators only allow the adhesive to bond with the dual cure composite, without transforming the adhesive into a self-curing one. Conversely, other dual-cure activators convert their adhesives into dual-cure adhesives. By recognizing these contrasts, and also the differences between light cure and dual cure materials, we can select the appropriate materials for our clinical circumstances, resulting in the finest restorations we can provide for our patients.